In pharmaceutical manufacturing, success does not begin with equipment — it begins with layout.
A well-planned facility flows like a controlled ecosystem. Materials move logically. People move safely. Air flows intentionally. Compliance is built into the walls.
Pharma facility layout and master planning is not architectural drafting. It is a strategic discipline that combines GMP regulations, production efficiency, contamination control, expansion strategy, and operational psychology.
If you are planning a greenfield pharmaceutical plant or expanding an existing site, this guide explores how intelligent master planning becomes your long-term competitive advantage.
- Why Pharma Layout Planning Is a Critical Investment
A poorly designed pharmaceutical facility leads to:
- Cross-contamination risks
- Regulatory observations
- Inefficient material movement
- Production bottlenecks
- Expansion limitations
- High operational cost
A properly designed pharma layout ensures:
Logical process flow
GMP compliance by design
Controlled contamination risk
Efficient manpower utilization
Utility optimization
Seamless scalability
Layout mistakes are expensive to correct after construction. Master planning prevents those mistakes.
- What Is Pharma Master Planning?
Master planning is the strategic blueprint of the entire pharmaceutical campus. It defines:
- Land utilization
- Building positioning
- Production zoning
- Utility corridors
- Traffic movement
- Future expansion areas
- Environmental compliance zones
It answers one fundamental question:
How will this facility operate efficiently for the next 20 years?
- Core Principles of Pharmaceutical Facility Layout Design
- 1️⃣ Unidirectional Material Flow
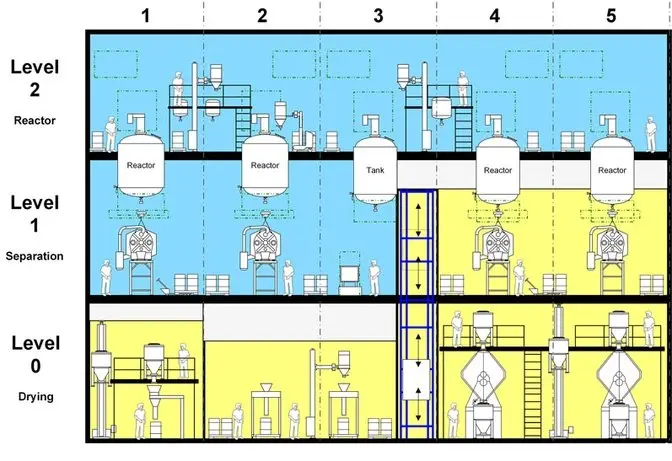
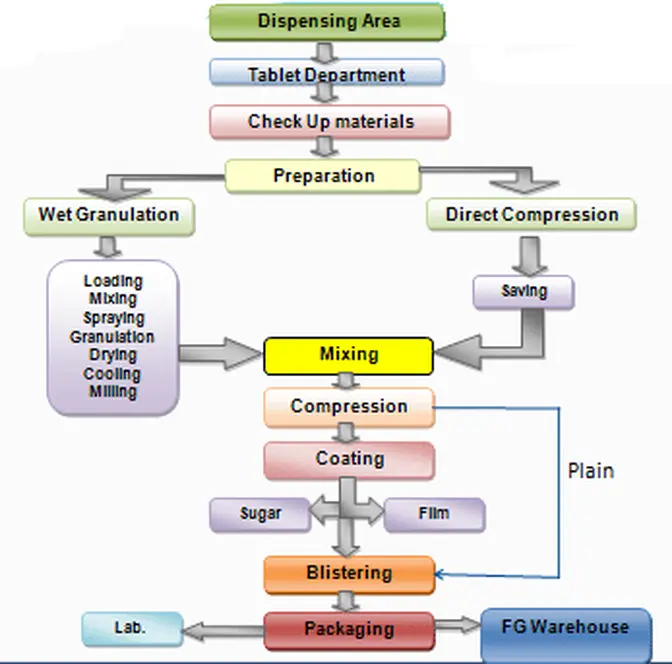
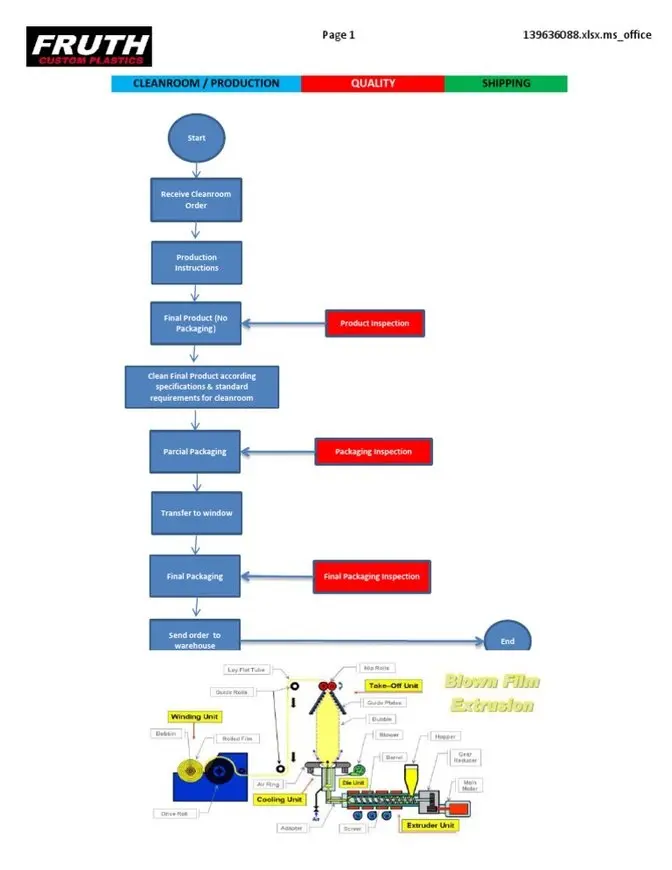
Raw materials should move forward — never backward.
Typical flow:
Raw Material Warehouse → Dispensing → Manufacturing → Packing → Finished Goods → Dispatch
Backtracking increases contamination and mix-up risk.
- Segregation Strategy
Pharmaceutical layouts must segregate:
- Raw vs finished goods
- Sterile vs non-sterile zones
- High potency vs general production
- Personnel vs material movement
- Waste exit vs product flow
Segregation is a GMP requirement, not a design preference.
- Zoning Based on Risk Classification
Different product types require different zoning:
OSD (Oral Solid Dosage)
Dust containment & pressure cascade control
- Sterile Injectable
ISO-classified cleanrooms & controlled access
- API Manufacturing
Hazardous area classification & solvent handling zones
- Biotech & Vaccine
Biosafety level segregation
Master planning integrates all risk zones into a unified campus structure.
- HVAC and Pressure Cascade Planning
Airflow defines compliance.
Layout must support:
- Proper air handling unit (AHU) placement
- Pressure differential zoning
- Return air strategy
- Clean corridor concept
- Service corridors
HVAC routing must be integrated at planning stage — not retrofitted later.
- Utility & Service Corridor Integration
Modern pharma facilities include:
- Dedicated technical corridors
- Interstitial service floors
- Maintenance access without production interruption
- Centralized utility backbone
Smart master planning reduces maintenance downtime.
- Greenfield vs Brownfield Pharma Layout Planning
- Greenfield Facility
- Full design flexibility
- Optimal zoning possibilities
- Easier regulatory alignment
- Higher capital investment
- Brownfield Expansion
- Space constraints
- Utility capacity limitations
- Validation challenges
- Operational continuity risks
A structured feasibility study helps determine expansion viability.
- Warehouse & Logistics Integration
Pharma layout must incorporate:
- Quarantine areas
- Sampling rooms
- Temperature-controlled storage
- Hazardous storage rooms
- Automated racking systems
- Separate dispatch zones
Logistics inefficiency silently reduces plant productivity.
Common Pharma Layout Mistakes to Avoid
Cross-over between personnel and material
Insufficient expansion planning
Poor waste exit planning
Oversized corridors without functional purpose
Ignoring maintenance accessibility
Improper cleanroom airlock positioning
Small design oversights create long-term compliance challenges.
- Future-Ready Pharma Master Planning Trends (2026 & Beyond)
Pharma infrastructure is evolving toward:
- Modular production suites
- Flexible multi-product facilities
- Digital twin layout simulation
- Energy-efficient campus planning
- ESG-compliant site design
- Automation-integrated flow modeling
Future-ready layouts support process upgrades without structural modification.
How Professional Pharma Layout Consultants Add Value
xperienced pharma layout consultants provide:
GMP-aligned architectural design
Risk-based zoning strategy
Regulatory inspection readiness
Process-driven layout optimization
Utility capacity forecasting
Long-term scalability planning
Their expertise reduces redesign costs and regulatory delays.
The Strategic Impact of Master Planning
A well-designed pharmaceutical facility:
- Reduces production cycle time
- Improves contamination control
- Enhances audit confidence
- Minimizes energy consumption
- Increases operational efficiency
- Strengthens long-term asset value
Master planning is not a cost — it is an investment in compliance and profitability.
Final Thoughts
Pharma facility layout and master planning determine how efficiently your plant will operate for decades.
Walls, corridors, cleanrooms, warehouses, and utilities must align with process flow and regulatory expectations from day one.
Whether you are planning:
- An OSD manufacturing plant
- A sterile injectable facility
- An API production unit
- A biotech or vaccine site
- Or a multi-product pharmaceutical campus
Strategic master planning ensures your facility is compliant, scalable, and future-ready.