The Story:
It’s early morning in a small café, and the head chef is preparing breakfast. She heats a pan on high heat to sear a steak. Before adding the oil, she wants to check if the pan is hot enough. She flicks a few drops of water onto the surface—and to her surprise, instead of instantly evaporating, the tiny droplets begin to dance, skimming across the pan as if they had a life of their own.
She watches closely. The droplets move around, shrinking ever so slightly, but they don’t disappear right away. Some even seem to float for a few seconds before finally vanishing into steam.
Curious, she calls over her apprentice:
"Why aren’t these water drops evaporating immediately? The pan is scorching hot—shouldn't they disappear in an instant?"
Question:
Why does water remain in droplet form for a short time on a very hot surface instead of evaporating instantly?
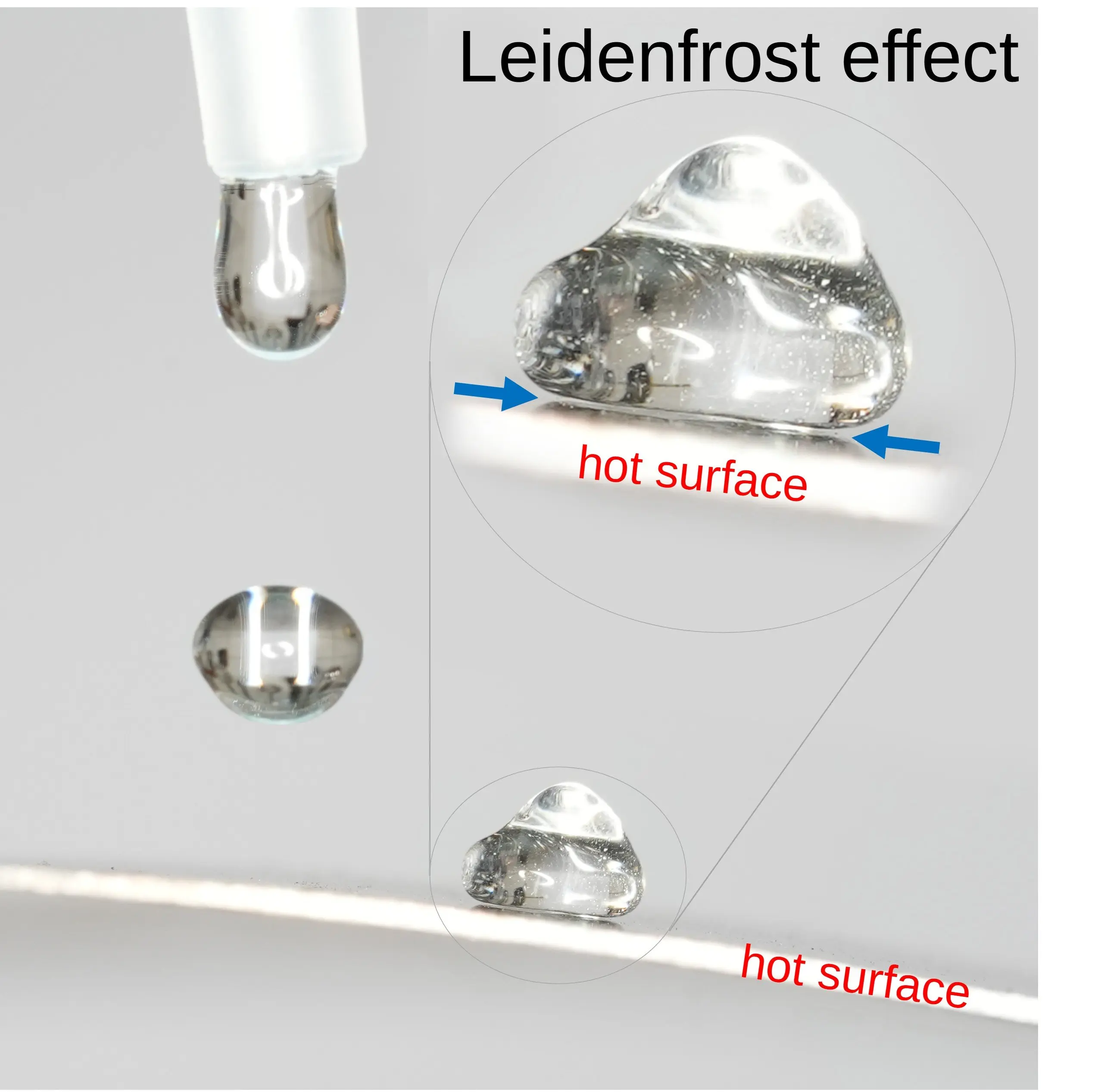
Dirk8B, CC BY-SA
The Phenomenon: The Leidenfrost Effect
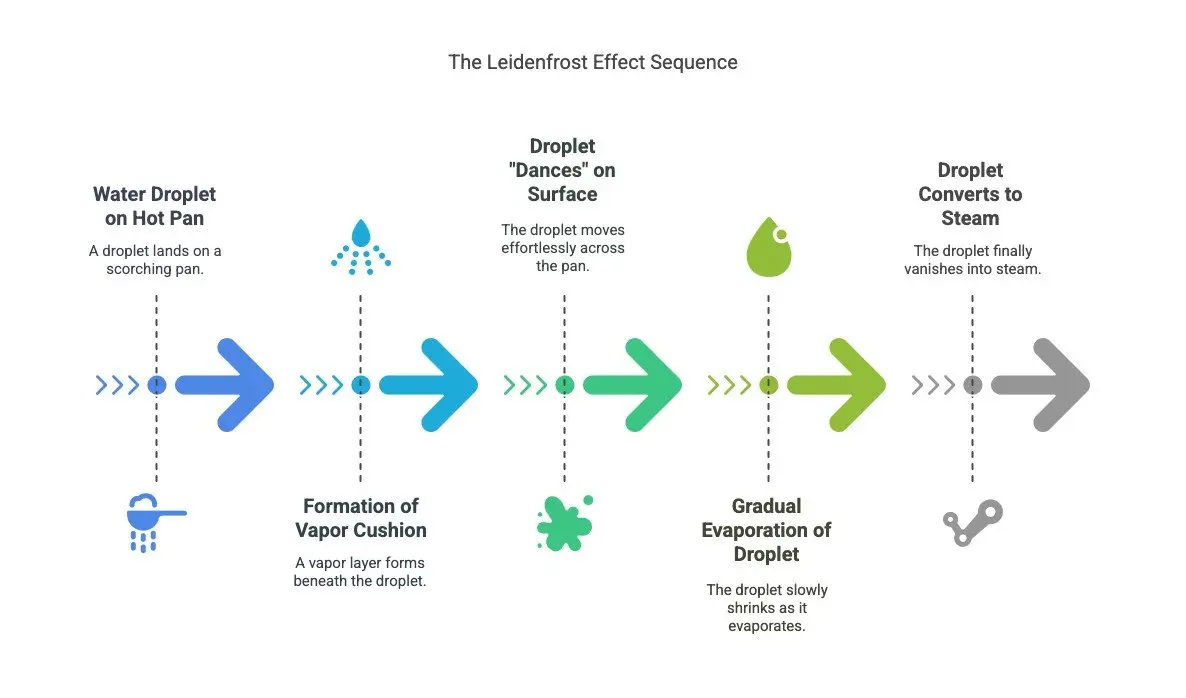
When water touches a very hot surface, something surprising happens: Instead of instantly evaporating, small droplets seem to “dance” on the surface. This is due to the Leidenfrost Effect, named after the German scientist Johann Gottlob Leidenfrost, who described this phenomenon in the 18th century.
What Exactly Happens?
- Temperature Differences Are Key
- If the pan is just slightly above 100°C (212°F), the water evaporates immediately.
- However, when the surface temperature exceeds around 200-250°C (392-482°F), something unusual occurs: The droplets no longer touch the hot surface directly.
- A Vapor Cushion Forms a Protective Layer
- The bottom layer of the droplet instantly turns into vapor, creating a thin insulating layer between the droplet and the pan.
- This vapor cushion acts like an air cushion, preventing the droplet from coming into direct contact with the hot surface.
- The Droplet "Floats" and Moves
- The vapor reduces friction between the droplet and the pan, allowing the droplets to move seemingly effortlessly.
Why Doesn’t the Water Evaporate Immediately?
- The vapor insulates the droplet from the hot surface, slowing down heat transfer.
- Only when the droplets shrink due to gradual evaporation do they eventually touch the hot surface and disappear into steam.
Try It Yourself: Leidenfrost Effect Experiment
You can easily observe this phenomenon at home:
- Heat a pan on high heat.
- Carefully drop a small amount of water onto the pan.
- If the pan is not hot enough, the water will evaporate instantly.
- If it’s hot enough, the droplet will "dance" around the pan.

Applications in Science and Technology
The Leidenfrost Effect is used in various fields:
- Coatings: Materials are designed to repel liquids, preventing direct contact.
- Optimized Cooling Systems: Certain technologies use this effect to improve heat dissipation.
Conclusion:
The dancing water droplet on a hot pan is a fascinating example of how physics influences our daily lives. And who knows—you may have seen this effect before without realizing why it happens!